Background
Patients with overt myelofibrosis (MF) suffer a significant symptom burden and impairment of health-related quality of life (HRQoL). However, very little evidence-based data is available on HRQoL and the symptom burden of patients with pre-fibrotic MF.
Aim
The primary objective of this study was to describe the HRQoL profile of patients with pre-fibrotic MF relative to patients with overt MF. The secondary objective was to examine disease-specific symptom prevalence in both groups.
Methods.
Baseline data from an ongoing prospective multicenter observational study by the GIMEMA Group were analyzed. The inclusion criteria of this study were: adult patients diagnosed with Philadelphia chromosome-negative myeloproliferative neoplasm (MPN), according to the 2016 WHO classification, within one year before the registration date. For the purpose of this analysis, only patients with pre-fibrotic and overt MF were considered. HRQoL profile was assessed with the EORTC QLQ-C30, which consists of 30 items and includes five functional scales (physical, role, emotional, social, and cognitive), three symptoms (fatigue, nausea, vomiting, and pain), a global health status/QoL scale and six single items (dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulties). Well-established evidence‐based guidelines for interpreting the QLQ‐C30 were used to determine clinically meaningful differences between groups (i.e., pre-fibrotic vs overt MF). Given the exploratory nature of the study, n o statistical testing was performed on mean score differences. Symptom prevalence was assessed by items of the MPN-SAF TSS. The MPN-SAF TSS is a comprehensive and brief 10-item instrument that concisely assesses the prevalence and severity of debilitating symptoms of MF. The percentage of patients who reported symptoms on the MPN-SAF TSS was based on the number of patients who answered at least 1 on a given item.
Results.
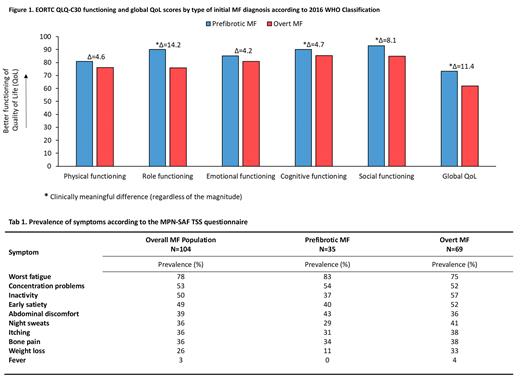
Overall, 104 patients with a median age of 68 years (IQR: 59-76) were enrolled across 20 community and university-based hospitals. More than half of the patients were male (n=58; 56%), and 55 (53%) revealed splenomegaly. Thirty-five patients (34%) reported at least one comorbidity. The median time since diagnosis for the overall group was 4 months (IQR: 1-7), and there were 69 and 35 patients diagnosed with overt and pre-fibrotic MF, respectively. The median age of patients with overt MF and pre-fibrotic MF was 69 (IQR: 61-76) and 63 (IQR: 57-75), respectively. At study entry, no statistically significant differences were observed between the two groups regarding age, sex, prevalence of comorbidities, WBC, and PLT counts. However, patients with overt MF had lower Hb levels (p=.003).
Inspection of the HRQoL profile, according to the EORTC QLQ-C30, showed that patients with overt MF had a statistically and clinically meaningful worse global HRQoL compared to patients' pre-fibrotic MF (Δ=11.4). The following functioning scales were also mostly impaired in patients with overt MF: role functioning (Δ=14.2), cognitive functioning (Δ=4.7), and social functioning (Δ=8.1) (Figure 1).
With regard to symptoms severity, patients with overt MF reported clinically meaningful higher severity for fatigue (Δ=6), pain (Δ=7.2), insomnia (Δ=6), constipation (Δ=5.4), and appetite loss (Δ=6.8). However, patients with pre-fibrotic MF reported clinically meaningful higher severity for dyspnea (Δ=5.5).
The prevalence of symptoms according to the MPN-SAF TSS, overall and by disease group, is reported in Table 1. The prevalence of symptoms in the overall group of patients was high, with the top three most prevalent symptoms being fatigue (78%), concentration problems (53%), and inactivity (50%). Notably, the prevalence of symptoms was also high in patients with pre-fibrotic MF, with some one-third of them reporting: fatigue, concentrations problem, inactivity, early satiety, abdominal discomfort, night sweats, itching, and bone pain.
Conclusions.
To the best of our knowledge, this is one of the first evidence describing HRQoL and the symptom burden of patients with pre-fibrotic MF. Although our preliminary data suggest that the HRQoL profile of patients with pre-fibrotic MF was generally worse than those with overt MF, our findings also indicate a high symptom prevalence in patients with pre-fibrotic MF.
Disclosures
Palandri:Novartis, BMS, Celgene, GSK, Amgen, AbbVie, Karyopharm, AOP, Sierra Oncology, Janssen: Consultancy, Honoraria. Patriarca:Alexion: Consultancy; Sobi: Consultancy, Speakers Bureau; Sanofi: Consultancy, Research Funding, Speakers Bureau; Pfizer: Consultancy, Speakers Bureau; Takeda: Consultancy, Research Funding, Speakers Bureau; Incyte: Consultancy, Research Funding, Speakers Bureau; Novartis: Consultancy, Research Funding, Speakers Bureau. Iurlo:Novartis, Pfizer, Incyte, BMS, GSK, AOP Health: Honoraria. Abruzzese:BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy; Takeda: Consultancy. Fozza:BMS: Research Funding; Amgen: Research Funding; Sanofi: Research Funding. Luppi:Novartis: Membership on an entity's Board of Directors or advisory committees; Jazz Pharma: Membership on an entity's Board of Directors or advisory committees; MSD: Membership on an entity's Board of Directors or advisory committees; Gilead Sci: Membership on an entity's Board of Directors or advisory committees, Other: Travel Grant; Abbvie: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees; Grifols: Membership on an entity's Board of Directors or advisory committees. Vignetti:Novartis: Speakers Bureau; AbbVie: Honoraria; Uvet: Honoraria; Dephaforum: Honoraria; ER Congressi: Honoraria; IQVIA: Honoraria. Vannucchi:Roche: Honoraria; Abbvie: Honoraria; GSK: Honoraria; BMS: Honoraria; Novartis: Honoraria; Incyte: Honoraria; AOP: Honoraria. Efficace:Syros: Consultancy; AbbVie: Consultancy; Incyte: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal